India is on the verge of approving Eli Lilly’s tirzepatide, a potentially groundbreaking drug already used for type 2 diabetes treatment in other markets, for weight loss. The Central Drugs Standard Control Organisation (CDSCO) has granted initial approval, although specifically for diabetes management, with further review underway for its weight-loss application.
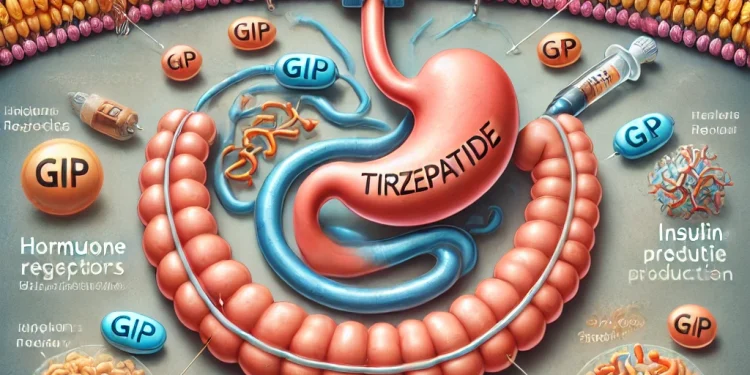
Mechanism of Action
Tirzepatide, marketed as Mounjaro for diabetes and Zepbound for weight loss, works by mimicking hormones GIP and GLP-1. This action stimulates insulin production, lowers blood sugar levels, and suppresses appetite, which is crucial for weight reduction among obese individuals.
Clinical Trials and Efficacy
Clinical trials have indicated impressive efficacy, with significant weight loss observed in participants. However, side effects such as gastrointestinal discomfort have been noted, underscoring the need for careful monitoring and adherence to prescribed usage.

Implications for India

Implications for India
The anticipated arrival of tirzepatide in India, possibly by 2025, holds promise in addressing the country’s high rates of diabetes and obesity. While not a cure-all, its approval marks a significant advancement in medical options for weight management.
Conclusion
The introduction of tirzepatide in India could revolutionize the treatment landscape for both diabetes and obesity. Continuous monitoring and responsible usage will be essential to maximize benefits and minimize risks associated with this new therapeutic option.